by Roger Kelly and Majid Ghoddusi
Fixation of any tissue for electron microscopy is best achieved by perfusion of the tissue with chilled buffered glutaraldehyde via its arterial supply. But to run in a large volume of expensive fixative via an aortic cannula is problematic and wasteful in animals and birds larger than rats. We have had success in chickens and dogs by injecting the fixative (10-20ml) just beneath the liver capsule using a fine flexible cannula.
A 3cm 23 gauge Record hypodermic needle is nicked with a capsule file at about half its length, and the needle is broken by bending back and forth through a shallow angle at the nick (the shallow angle ensures that the lumen is not burred over and occluded). The proximal end of the distal fragment is inserted into thin polythene tubing (about 10cm long) just wide enough to allow the blunt needle end to be inserted. The join should of course be completely water-tight. The other end of the tube is then forced over the stub of the needle protruding from the Record fitting.

Birds were anaesthetised by intramuscular injection of a mixture of ketamine (25mg/kg) and xylazine (20mg/kg). This gives you plenty of time to expose the liver without the blood pressure falling. Having an assistant is important because the sternum needs to be held up out of the way. The fixative of choice (we used 3% glutaraldehyde in 0.066m cacodylate buffer at pH 7.2) has beforehand been prepared fresh from stock solutions and drawn up into a 10ml syringe.
The needle is gently slipped through the liver capsule at a fairly narrow angle, and left there without trying to hold it in place. The flexible plastic tubing allows injection of fixative to be made without any disruptive movements of the needle, which would be inevitable if the needle was directly connected to the syringe.
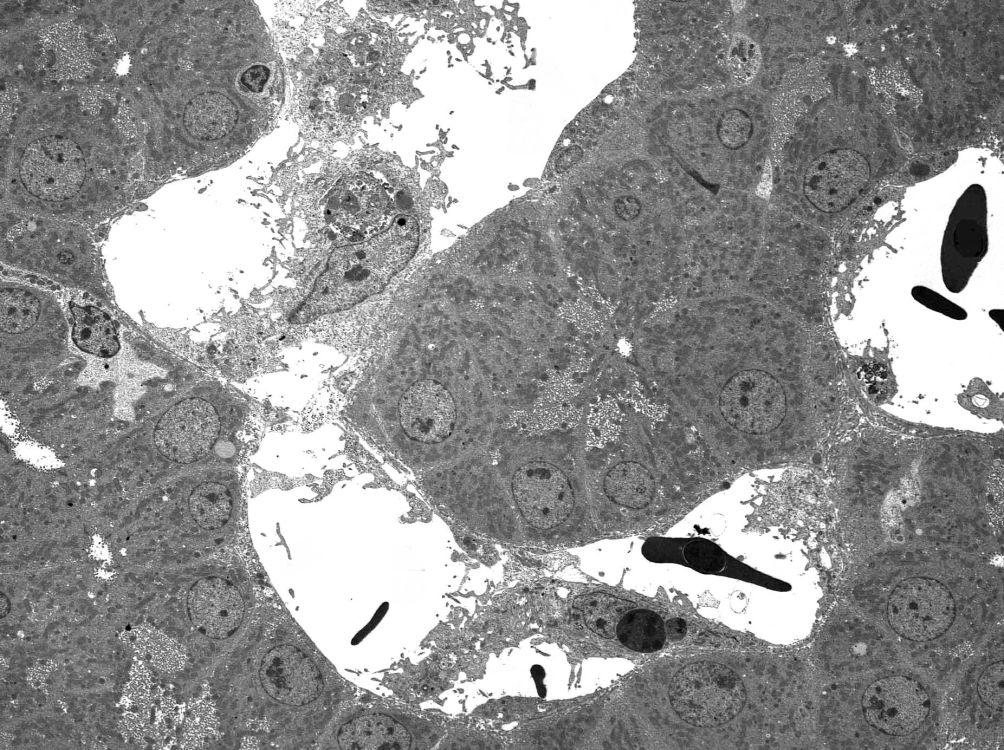
The idea is to be able to see the change in the parenchyma around the needle as you inject, so you don’t want the needle-tip diving down too deep. This would also increase the chance of the fixative entering a larger vein and bypassing the sinusoids.
The pressure used to inject the fixative was not critical. If you are too strong, you will see locally disrupted parenchyma on slicing the perfused tissue; in this case, make sure you select a site a bit further away from the needle entry site that shows the firm, homogenous yellow appearance of glutaraldehyde-fixed liver. We just pushed it in at a rate of about 5ml/min and hoped to see an immediate yellow firm zone spread out from the injection site. As soon as the 10ml was gone, we killed the bird with barbiturate I/V and carefully cut out the perfused area as a 1cm cube and sliced it into 1mm slices into a puddle of cold fixative on a concave wax board. These 1cm slices can be quite large in area provided that they are no more than 1mm thick. If you use a magnifying loupe, you can select the most promising bits of these slices and cut them out and fix them some more (an hour or so in the cold) before osmicating and further processing.
The same technique can of course be adapted for use on any creature with a liver.